Human NK cells are known to contain subsets of CD56 +CD16 + and CD56 +CD16 - cells. The former directly attack target cells by an antibody-dependent cellular cytotoxicity (ADCC) various organs in the body, whereas the latter are mainly found in lymph nodes and activate inflammatory and immune responses by secreting IFNγ. However, despite their importance in the biological defence systems, whether and how these subsets emerge is not yet fully understood. In particular, several recent studies have challenged the conventional linear differentiation model which positioned CD56 +CD16 - cells as progenitors of CD56 +CD16 + cells. However, during the stepwise fate specification from immature multipotent progenitors to NK cells, it is still unknown at which stage the final CD16-expressing capacity is determined and what stimuli are involved in this decision. Through cell fate tracking in human HPC-derived differentiation, present study has demonstrated that fate specification into distinct progenitor cells specific for the two subsets occurs at a stage before the cells have yet to express CD56. Furthermore, it showed that IL-4 has a positive effect, both in bona fide and PSC-derived hematopoiesis, on the fate decision to progenitor cells from which CD56 +CD16 + NK cells emerge. While IL-4 was previously known to act on NK cells after CD56 expression, the present results show that IL-4 is also involved in future fate decisions at a more earlier progenitor cell stage than previous reports.
We first identified three CD34 -CD56 - NK progenitor cell fractions, which were clearly distinguished due to the intensity of CD117 expression (CD34 -CD56 -CD117 ++, CD34 -CD56 -CD 117 +, CD34 -CD56 -CD117 -) in the differentiation pathway from lineage-negative cord blood progenitors. Tracking the cell fate of each fraction showed that they all produced CD56 + NK cells, but that CD16-expressing cells emerged predominantly from the CD34 -CD56 -CD117 + and CD34 -CD56 -CD117 - fractions at a rate of 15-30%. In contrast, NK cells originating from the CD34 -CD56 -CD117 ++ fraction hardly expressed CD16.
Based on expression profile comparisons, we next identified IL-4 as a factor involved in progenitor cell fate decisions. IL-4 stimulation during early stage differentiation increased the CD34 -CD56 -CD117 + and CD34 -CD56 -CD117 - fractions, and conversely reduced the CD34 -CD56 -CD117 ++ cells. As a result, not only was the emergence efficiency of CD16-expressing NK cells increased, but their cytotoxic potential was also enhanced. During this process, IL-4 enhanced EOMES expression, which was in common with previous reports on T cells.
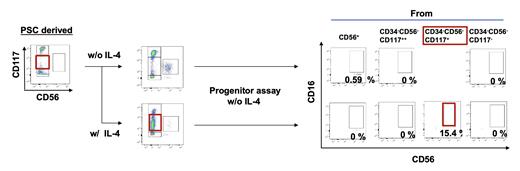
Finally, we investigated whether NK cells derived from human pluripotent stem cells (hPSCs) also develop along the same trajectory as bona fide hematopoietic cells. Using a refined system of our previously reported NK cell differentiation cultures, we found that, as in cord blood, many hPSC strains produced all of three progenitor cell fractions, and both CD16 + and CD16 - NK cells appeared at the end of culture. In contrast, some strains produced only CD16 - NK cells, which were nearly deficient in the CD34 -CD56 -CD117 + fraction on the way of differentiation. Interestingly, stimulation with IL-4 significantly reduced the proportion of CD34 -CD56 -CD117 ++ progenitor cells from those strains (52.8±14.4% to 10.4 ±4.7%), which instead also produced CD34 -CD56 -CD117 + progenitors (13±3.4% to 68.1 ±8.3%) from which around 15% of CD16 + NK cells were eventually generated (Figure).
Our results support a branching model in human NK cell differentiation, no matter whether they are derived from cord blood or hPSCs. We found that IL-4 stimulation accelerates specification into the progenitors capable of producing CD16 + NK cells in the future at a much earlier stage than previously thought. Furthermore, we showed that IL-4 could correct strain-dependent differentiation propensities of hPSCs to stably induce functional NK cells with high ADCC activity.
Disclosures
No relevant conflicts of interest to declare.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal